Understanding Why Time Moves Forward: An Exploration of Entropy
Written on
Chapter 1: The Nature of Time
Why does time only seem to advance in one direction? While this question appears straightforward, it is surprisingly complex, and no conclusive answer exists. Let’s delve deeper into this intriguing topic.
This paragraph will result in an indented block of text, typically used for quoting other text.
Section 1.1: Perspectives from Physics
From the standpoint of various physical theories—ranging from Newtonian mechanics to Einstein's relativity and even quantum mechanics—there is fundamentally no difference in the direction in which time flows. The laws of physics remain consistent regardless of whether time is perceived as moving forward or backward.

Subsection 1.1.1: The Second Law of Thermodynamics
However, there is one notable exception: the Second Law of Thermodynamics. This principle states that in a closed system, the entropy will either increase or remain constant over time. While entropy is often associated with disorder, it is more accurately viewed as a measure inversely related to the thermal energy available for performing work. A system rich in usable energy is considered low-entropy, whereas a system with little available energy is high-entropy. Thus, the Second Law of Thermodynamics uniquely indicates a preferred direction for the flow of time.
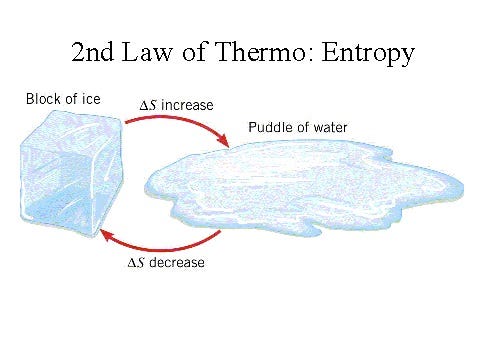
Section 1.2: Everyday Examples of Entropy
In real-life scenarios, this principle manifests in familiar ways: scrambled eggs cannot revert back to their original state as eggs, ice in a drink cannot re-form once melted, and coffee mixed with milk will not spontaneously separate again. These examples illustrate how low-entropy states inevitably transform into higher-entropy states over time.
Chapter 2: The Universe and Entropy
When we view the universe as a closed system, the Second Law of Thermodynamics implies that its entropy must continually rise. This principle elucidates many observable phenomena, such as the inability of mixed substances to revert to their separate forms.
The first video titled "Why Does Time Go Forward?" explores the fundamental reasons behind the unidirectional flow of time, giving insight into the subject.
On a cosmic scale, the universe began in a state of extremely low entropy, originating from the Big Bang when all matter and energy were concentrated in a minuscule point. This state was characterized by immense density and heat, leading to very low entropy.

Following the Big Bang, the universe expanded rapidly, and its entropy increased—a process that continues to this day. According to the theory of thermal death, the universe will eventually reach a state of thermodynamic equilibrium, at which point time as we understand it will cease to exist. The universe will become an expanse of emptiness, with all stars extinguished and black holes dissipated, marking the end of time's arrow.
The second video titled "The Time-Reversibility Paradox - Why Time Flows Both Ways" dives into the complexities surrounding the flow of time and its implications for our understanding of the universe.
The existence of stars, galaxies, and life on Earth serves as evidence of the universe's initial low-entropy state. Therefore, time flows forward as every closed system gravitates towards thermodynamic equilibrium. The idea of reversing time would require the universe to transition from a higher entropy state to a lower one, which is fundamentally impossible.
If you enjoy learning about space, be sure to subscribe to our channel for more content and feel free to ask any questions that will be addressed in future articles!